First Commercial Duchenne Muscular Dystrophy Therapy in US Administered at UF Health
For media inquiries, call Rossana Passaniti at 352-273-8569 or email passar@shands.ufl.edu
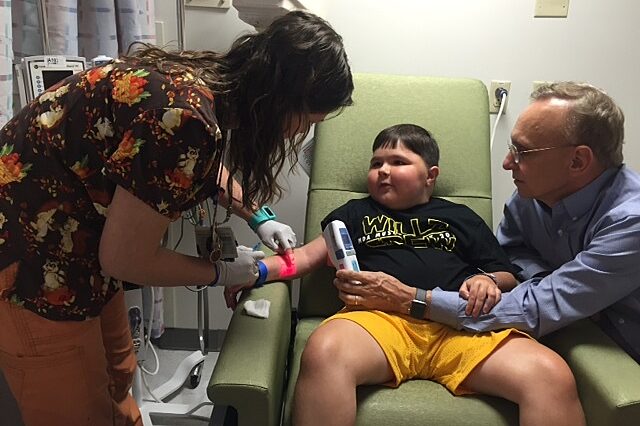
The first commercially available treatment in the United States for patients with Duchenne muscular dystrophy was administered on Wednesday at UF Health Shands Children’s Hospital.
Duchenne muscular dystrophy is the most common form of muscular dystrophy and primarily affects males. Symptoms of the progressive muscle-wasting condition appear in early childhood and usually lead to severe disability in the teenage years and death in the mid to late 20s.
The therapy, approved just last week, called Exondys 51 was given to a 9-year-old patient from Jacksonville at the infusion center at UF Health Shands Children’s Hospital. The drug is administered via weekly infusions and is considered to be personalized medicine because it is targeted at a specific genetic mutation. Around 13 percent of Duchenne patients worldwide are affected by the specific mutation addressed by this therapy.
Exondys 51, developed by Sarepta Therapeutics, became the first commercially available therapy in the U.S. for Duchenne after being granted accelerated approval by the Federal Drug Administration. The approval was based on an increase in dystrophin protein in skeletal muscle observed in biopsies of 12 patients in an initial trial, as well as those from 13 patients in an ongoing second confirmatory trial. The absence or deficiency of dystrophin is the underlying cause of the disease. Continued approval for Exondys 51 may be contingent on verification of clinical benefit in confirmatory trials.
“It is an honor to care for the first patient to receive a commercially available dose of this important new therapy,” said Barry Byrne, M.D., Ph.D., a professor of molecular genetics at the UF College of Medicine and the director of the Powell Center for Rare Disease Research and Therapy. Byrne is also the treating physician for one of 12 patients in the original human study of Exondys 51, previously called Eteplirsen. That patient has been receiving weekly infusions at UF Health for over three years.
“Access to Exondys 51 has been long awaited by patients and their families and this marks an important milestone for the field of personalized genetic medicine. This therapy is a culmination of decades of work by basic science and clinical researchers around the world,” said Byrne.