UF Health researchers’ gene therapy for eye disorder shows promise in early testing
For a video about how AAV gene therapy works, visit https://bit.ly/3J838qz.
GAINESVILLE, Fla. — For people with retinitis pigmentosa, the world they see often starts changing in childhood or as young adults. Their night and peripheral vision declines, eventually leaving most blind.
Now a group of University of Florida College of Medicine researchers are working on a solution: a gene therapy that has now succeeded in three animal models.
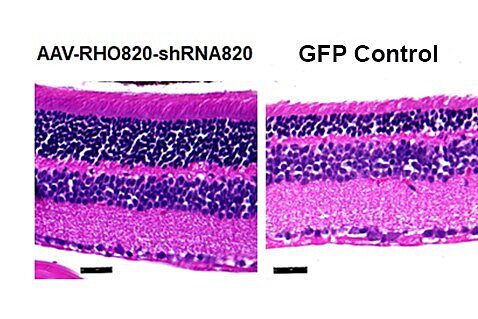
Working in mice, the researchers used gene therapy to both knock down and replace malfunctioning genetic material that affects light-sensing photoreceptors in the retina. The technique deploys a harmless, engineered virus to deliver a functional copy of the gene to the eyes. The therapy led to improved retinal structure and function throughout the nine-month experiment. The findings were published recently in the journal Vision Research.
The findings are significant because they show that the therapy works in different species. That is crucial to advancing it to human clinical trials, said Alfred S. Lewin, Ph.D., a professor emeritus in the College of Medicine’s department of molecular genetics and microbiology. The College of Medicine is part of UF Health, the university’s academic health center.
The therapy works by addressing unique mutations in the gene for rhodopsin, a protein crucial to the eye’s light-sensing system. It should work for the 100-plus rhodopsin mutations known to exist, Lewin said.
“The objective was to confirm that this therapy, which was tested previously in other animals, works in a different species with a different mutation. We also wanted to show that the therapy could have benefit after retinal degeneration has already started,” Lewin said.
In both cases, the answer was definitive. Mouse eyes treated with the gene therapy retained half of their retinal thickness — a key measure of preserving photoreceptors — after nine months, the researchers found. The eyes in untreated mice lost 40% of their retinal thickness after just one month and half of their photoreceptors in three months.
That, Lewin said, gives researchers reason to believe the therapy could work long term for humans. A genetically inherited disorder, retinitis pigmentosa typically strikes humans in the first or second decade of life. It affects one of every 5,000 people worldwide, according to the National Institutes of Health. There is no cure and no approved treatments for retinitis pigmentosa.
“Our ultimate goal would be to treat patients as early as we can — even before vision is lost — and while they can still learn to read and recognize the world around them,” Lewin said.
The researchers also made an unexpected discovery during the experiments: Treating one eye with the gene therapy also delivered some beneficial DNA to the animals’ untreated eyes, although at lower levels than the treated eyes. Among mice that received the therapeutic virus, the survival of photoreceptors was improved at all ages and in both eyes.
The researchers are encouraged by the bilateral benefit even if the mechanics of how an untreated eye benefits from treatment in a companion eye aren’t yet fully understood, said Chulbul M. Ahmed, Ph.D., a research assistant professor in the department of molecular genetics and microbiology and a co-author of the paper.
“You don’t necessarily have to treat both eyes. With treatment in one eye, the counterpart eye appears to get some benefit from that,” Ahmed said.
The work in mice also established that the therapy can be effective even when retinitis pigmentosa has taken hold.
“We wanted to see if we could treat an animal-model patient where degeneration had begun. In fact, we did get benefits in both eyes, even after the disease had already started,” Lewin said.
The researchers will now try to determine the smallest dose that is effective in certain preclinical models, Lewin said. That will help them approximate a dose that is safe for humans, which is another key step before moving into clinical trials.
Research funding was provided by the National Eye Institute, the National Institutes of Health, Research to Prevent Blindness, the Jewish Heritage Fund for Excellence, the Shaler Richardson Professorship Endowment and the Kentucky Lions Eye Research endowed chair. A University of Louisville researcher collaborated on the study.
About the author
