UF Anesthesiology teams up with wind engineering laboratory to make economy face shields
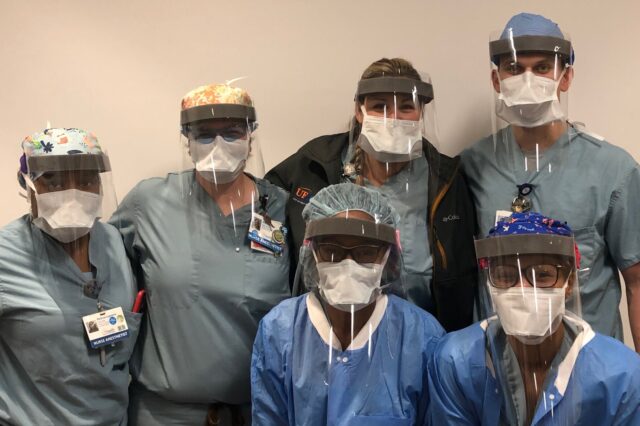
Aiming to help front-line health care workers from exposure to COVID-19, the University of Florida College of Medicine’s department of anesthesiology has teamed up with UF’s wind engineering laboratory to produce simple face shields and intubation technology when supplies of personal protective equipment were unknown in early April.
Using a 5-foot by 10-foot table computer numerical control, or CNC, router and a cutting knife, the UF Powell Family Structures and Materials Laboratory can make thousands of plastic face shields, each in less than one minute. The shields are worn over a mask or respirator to give health care workers added protection for their eyes, face and skin.
A team of anesthetists, administrative personnel and physicians assembled the shields using a self-adherent wrap material already available in the hospital for the straps and a piece of foam between the forehead and the shield to hold it off the face. Local Home Depot employee Charlie Gunnett, the husband of Amy Gunnett, R.N., CCRC, interim manager of clinical research in the department of anesthesiology, facilitated a donation of the foam and glue from the local Home Depot for the first batch.
“They’re a simple shield. They’re super quick and easy to make,” said Scott Powell, operations manager for the laboratory, which typically studies how wind interacts with man-made structures and builds models to test in hurricane research. “We are repurposing a lot of our fabrication ability to help support the medical community.”
Stephanie Gore, M.S.N., R.N., CCRN, the department of anesthesiology’s patient safety and quality officer, and Nik Gravenstein, M.D., the Jerome H. Modell M.D. Professor of Anesthesiology, collaborated with the Powell laboratory to use a simple, commercially made shield as a model.
“We got creative at a time when the availability of personal protective equipment was unknown to make sure all of the people on the frontlines are safe,” Gore said.
Powell was able to leverage his team of engineers and machinists, who specialize in quick design and fabrication for wind engineering. “We’re uniquely set up so that we can quickly respond to challenges if needed,” he said.
Using designs by Cameron R. Smith, M.D., Ph.D., an assistant professor of anesthesiology, and Patrick Tighe, M.D., M.S., an associate professor of anesthesiology, the Powell laboratory was also building an aerosol plexiglass tray to fit on a bedside table or Mayo stand that holds surgical instruments and materials. The see-through tray, which can be sterilized, extends over a patient’s head and is covered by a surgical drape for use during intubation.
These tools are used to help protect doctors in the hospital during intubation, allowing them to visualize the patient’s airway while aiming to minimize exposure to COVID-19 in extreme cases.
The laboratory has also reverse-engineered face shields for powered air-purifying respirators, known as PAPRs, and controlled air-purifying respirators, known as CAPRs, with assembly help from the department of anesthesiology. These respirators aim to provide advanced protection from airborne particles for frontline health care workers.
The Powell laboratory was also providing the plastic see-through foil and 3D-printed parts for another type of face shield called Prusa. They were working with other UF groups to print rapid-prototyped 3D parts.
Both Powell and Gore praised the unique collaboration between departments with different skill sets. “It’s been a great working relationship,” Powell said.
Although the face shields are not FDA-cleared or -approved, the FDA has authorized their use by health care providers as personal protective equipment under an FDA Emergency Use Authorization, or EUA. The EUA extends for the duration of the declaration that circumstances justifying the authorization of emergency use under Section 564(b)(1) of the Act, 21 USC 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
About the author
