The 21st Century Cures Act
In recent weeks, the 21st Century Cures Act was passed overwhelmingly by both chambers of the U.S. Congress. The Senate passed the bill on Nov. 30 by a vote of 94-5, and the House passed an almost identical version on Dec. 7, by a vote of 392-26. On December 13th, President Obama signed the bill into law.
More than two years in the making, this $6.3 billion legislation has been extraordinary in garnering bipartisan support from a Congress that has often been unable to act because of partisan gridlock. That said, the sausage-making process that is typical of any legislation appears to have been particularly noteworthy here: A bill that was originally 300 pages grew to almost 1,000 under the influence of a multitude of ardent supporters and critics, 1,300 lobbyists (including universities as well as the pharmaceutical and device manufacturing industries), pragmatic legislators and their staff, and the presidential election results.
Advocates frame benefits of the 21st Century Cures Act in terms of advancing research on Alzheimer’s disease, cancer and mental health, while breaking down regulatory barriers and expediting the approvals for drugs and devices. (For example, see these remarks by Fred Upton (R-Michigan), chair of the House Energy and Commerce Committee.) Detractors have commented that the amount of National Institutes of Health funding included in Cures does not represent a significant increment and must be reauthorized every year, and that drug and medical-device approvals based on “real-world” patient outcomes (rather than randomized clinical trials) could endanger patients because of incorrect conclusions about safety and efficacy. (For example, see this article in Fortune magazine.)
The 21st Century Cures Act will have broad ripple effects on research and patient care throughout the nation and at UF Health for years to come. While a comprehensive summary can be found here for those interested in the details, the goal of this edition of OTSP is to identify the key provisions and provide some context.
The 21st Century Cures Act contains $4.8 billion in spending over 10 years to establish an NIH Innovation Account for new research at the NIH, including:
- $1.8 billion for the cancer research “moonshot” championed by Vice President Joe Biden.
- $1.6 billion for the BRAIN Initiative, a project to create new technologies that will allow for comprehensive mapping of the human brain.
- $1.4 billion for the Precision Medicine Initiative, which will collect genetic data on 1 million American volunteers that will be used to help develop new treatments.
The nation’s prescription drug abuse crisis also is addressed through grants to states. These grants, worth $1 billion over the next two years, will be awarded to deliver drug abuse prevention and treatment programs. Moreover, there are several provisions affecting mental health: New positions in mental health training programs and demonstration projects for psychiatric and psychological treatment will be funded; enforcement of mental health parity requirements will be strengthened; and the Medicaid “same day” exclusion, which prohibits separate payment for mental health and primary care services provided to a Medicaid enrollee on the same day, will be eliminated. New positions at the U.S. Department of Health and Human Services will be also established to coordinate mental health and substance abuse research and treatment.
A large number of provisions in the 21st Century Cures Act are aimed at swift approval of new drugs and devices. About $430 billion is allocated over 10 years to allow the U.S. Food and Drug Administration to:
- Rely on data summaries and “real-world evidence” instead of the results of randomized clinical trials when weighing the approval of existing drugs for new uses.
- Use a “limited population” approval pathway for new antibiotics that would rely on a risk-benefit analysis weighing the needs of patients facing severe and untreatable infections against the possible harms to them.
- Expand its programs for expedited approval of breakthrough medical technologies for patients with life-threatening diseases that have limited treatment options.
- A variety of other provisions direct the FDA to address its regulatory infrastructure with the intent of reducing regulatory burden.
Many patient advocacy groups have applauded these new measures as a means of expediting new treatments that might be helpful for patients with serious illness, even if the data on safety and efficacy have not been obtained at the most rigorous standard. Critics, however, have expressed concern that these new regulatory provisions reflect extensive lobbying by pharmaceutical and medical device firms; they argue that the new approval guidelines are too lax and could raise the risk of harmful treatments getting to the marketplace.
Also under the banner of reduced regulatory burden is a provision that “requires the NIH Director, the Secretary of Agriculture, and the Commissioner of the FDA to review and revise as appropriate laboratory animal regulations and policies to reduce administration burden on investigators.”
Other provisions of the 21st Century Act strengthen privacy protections for genetic research participants; promote more pediatric research; enhance training programs and loan forgiveness for “next generation researchers”; improve the usability of electronic health records; recognize the role of socioeconomic status in the Medicare hospital readmissions reduction program; clarify that off-campus teaching hospital outpatient departments under development should continue to receive Medicare outpatient payment rates; and strengthen the FDA’s ability to hire, train and retain experienced staff scientists.
Payment for the provisions of the 21st Century Cures Act comes from two primary sources: rescinding funds from the Prevention and Public Health Fund, which was part of the Affordable Care Act, and the sale of crude oil from a portion of the Strategic Petroleum Reserve. According to the House Committee on Energy and Commerce, money from selling oil would go toward NIH funding “because just as energy reserves are a national resource designed to protect and serve our citizens, so too is an investment in health innovation and research.” Many in the public health community have decried the use of the PPHF to pay for the 21st Century Cures Act, but the reality of the situation is that the PPHF is part of the Affordable Care Act, and is among the ACA provisions that would be the first to go, with or without the 21st Century Cures Act.
All of the major professional medical associations (e.g., Association of American Medical Colleges, American Medical Association) have issued statements in support of the Act, as have most specialty and patient advocacy societies (e.g., American Neurologic Society, American Cancer Society, American Psychological Association, American Psychiatric Association, American Society for Clinical Oncology, American Society of Human Genetics, the Coalition to Stop Opioid, and the American Heart Association). Some of these statements express caveats about rescinding funding from the Prevention and Public Health program, the need for annual renewal by Congress for the NIH research funding, and the new guidelines for evidence of safety and efficacy.
Let’s try to put this into context. First, as can be seen in the graph below, the level of NIH funding nationally received a welcome boost in 2016, after being flat for the previous eight years. Counting this 2016 increase, the NIH budget increased about 19 percent in nominal dollars from 2003 to 2016, while declining about 19 percent in inflation-adjusted dollars during that period.
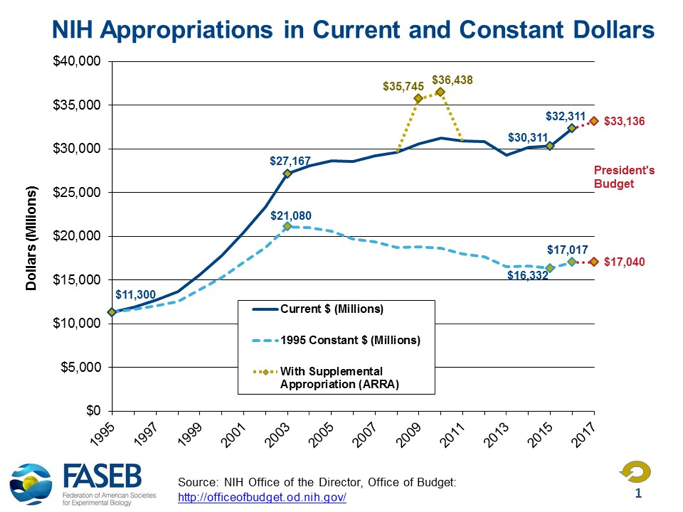
Therefore, to support our nation’s efforts in basic, translational and clinical research, any increase in NIH funding will be helpful. Establishing the NIH Innovation Account under the 21st Century Cures Act amounts to a boost of $480 million per year for 10 years. While certainly welcome, this increase in funding is only about 1.5% of the 2016 base, well below the inflation rate for conducting biomedical research. We can only hope that Congress will also pass the full FY 2017 NIH appropriations of $34.1 billion before January and avoid the delays and inefficiencies that further “continuing resolutions” impose. Put differently, it is important that the NIH Innovation Account augments, rather than supplants, the base NIH appropriation in FY 2017 and beyond. As noted by the AAMC, augmentation “is consistent with the intent of the original legislation and subsequent iterations, and is especially important since funding in the Innovation Account is half of the level originally approved in H.R. 6.”
Second, there is no question that obtaining FDA approval of a new drug, whether it be a new molecular entity approved through a new drug application or a new therapeutic biologic approved through a biologic license application, can be extremely challenging. For this and other reasons, there is a perception that regulatory factors have contributed, across time, to a significant diminution of drugs getting to market. This perception does not square with the data, however. As can be seen in the figure below, the number of drugs approved by the FDA was between 20 and 30 per year between 2002 and 2011. In 2014 and 2015, however, there were a record number of FDA approvals — 41 and 45, respectively. This increase in approvals has been due to a variety of expedited programs developed by the FDA (fast track, breakthrough therapy, priority review and accelerated approval) and more applications for orphan drugs (for conditions affecting less than 200,000 Americans) as opposed to first-in-class drugs (i.e., agents with a unique mechanism of action). Orphan drugs accounted for about half of the approvals in 2014 and 2015.
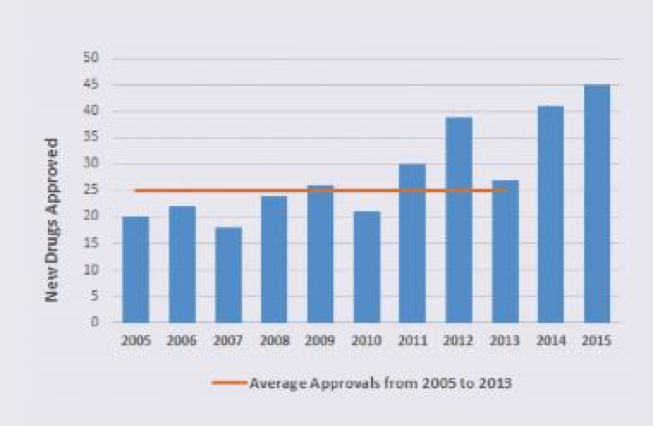
All that said, the cost in time and dollars to obtain FDA approval for a new drug is daunting and no doubt inhibits innovation and risk-taking. According to a study from Tufts University published in the May 2016 issue of the Journal of Health Economics, it takes on average more than a decade and $2.6 billion to bring a new compound to market, and an additional $300 million in required post-approval studies, for a total of $2.9 billion. These are daunting figures indeed, especially given that the overall probability of success in moving from Phase 1 trials to approval is only 9.6 percent, based on a recent large study.
This is where the rubber meets the road. How can we strike the right balance between wanting to speed biomedical discoveries to translational reality and patient benefit while avoiding the too-quick approval and marketing of drugs and devices that are of no benefit compared to placebo, or worse, are harmful?
The aspiration behind the 21st Century Cures Act can be found in the language of its supporters on the House Energy and Commerce Committee — a desire to bring “health care innovation infrastructure into the 21st century, delivering hope for patients and loved ones and providing necessary resources to researchers to continue their efforts to uncover the next generation of cures and treatments. In the 21st Century, health care innovation is happening at lightning speed. From the mapping of the human genome to the rise of personalized medicines that are linked to advances in molecular medicine, we have seen constant breakthroughs that are changing the face of disease treatment, management and cures. Health research is moving quickly, but the federal drug and device approval apparatus is in many ways the relic of another era. We have dedicated scientists and bold leaders at agencies like the NIH and the FDA, but when our laws don’t keep pace with innovation, we all lose.”
Let us hope that those charged with implementation of cures hold true to these aspirations, and ensure that expedited innovation is safe and effective innovation.
The Power of Together,
David S. Guzick, M.D., Ph.D. Senior Vice President for Health Affairs, UF President, UF Health
About the author
